|
|

|
This tab holds all of the documents, that you require, for the enquiry, each document tab has a 'traffic light' flag which shows when the document has been created as a PDF (green) or if the document will be created when you access it (red). We can change (subject to legal constraints) the look of the document or create new documents for your company.
Under EU law you have to keep each MSDS revision you make for 5 years so we recommend that you flag the MSDS's to be created as PDF's every time you authorise a formulation.
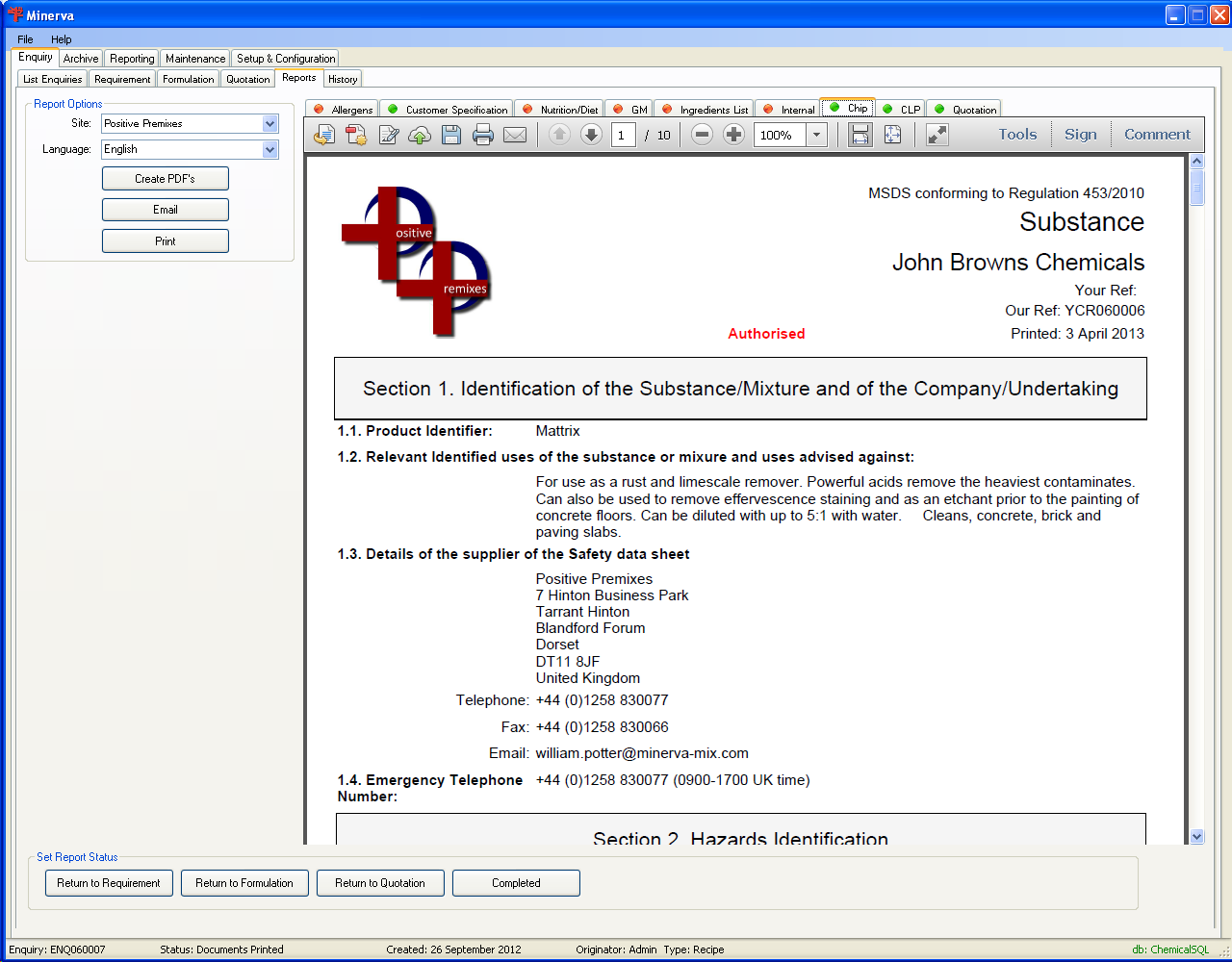
| Report | Description |
| Allegens | Allegen statement for the formulation. Conforms to Annex IIIa of EU Directive 2000/12/EC |
| Customer Specification | Confirmation of your understanding of the customers requirements |
| Nutrition/Diet | The nutritional and dietary statement as required by the EU directive 90/496/EEC. |
| GM | The GMO declaration as required by EU Directive 2001/18/EEC |
| Ingredients List | The list of ingredients for the formulation. |
| Internal | The composition of the formulation to be used in the manufacture. |
| Chip | This is the Chip (DPD) MSDS for the formulation. Since June 2015 this must NOT be supplied within the EU and under the EU regulations all previous changes to this document must be held for 5 years. |
| CLP | This is the GHS/CLP MSDS for the formulation. This must now be supplied with all formulations manufactured since 1st June 2015. This document replaces both DSD/DPD versions of MSDS because the EU regulations have been repealed. Under EU regulations all changes to this document must be held for 5 years. When you update the formulation or change how hazardous a substance is you must supply the new version to the customers that have had this formulation in the previous 12 months. |
| Quotation | The quotation for the formulation. |
Using the report options you can:
| Site | This will default to the site selected on the requirements screen, but you can change it for the print-outs here. |
| Language | This will default to the language selected on the requirements screen. This allows you to create a formulation in one language and then output it in any Minerva known language. |
| Create PDF's | Select the reports and the language then click the button. The selected reports will now be produced as PDF's. 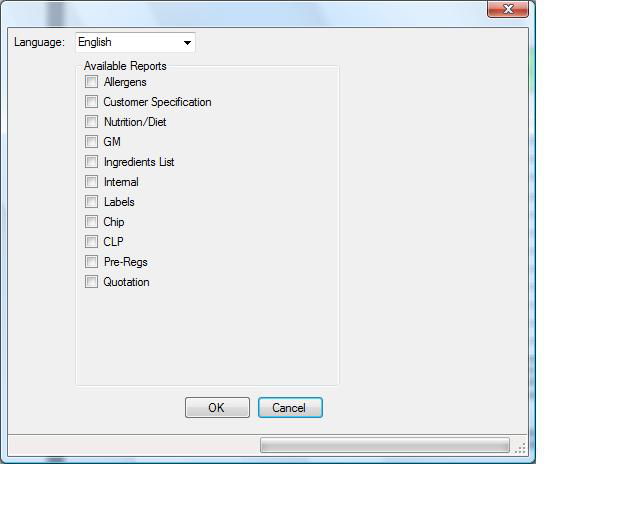 |
Select the reports to be emailed, then click the button, an email will also be sent to your email. 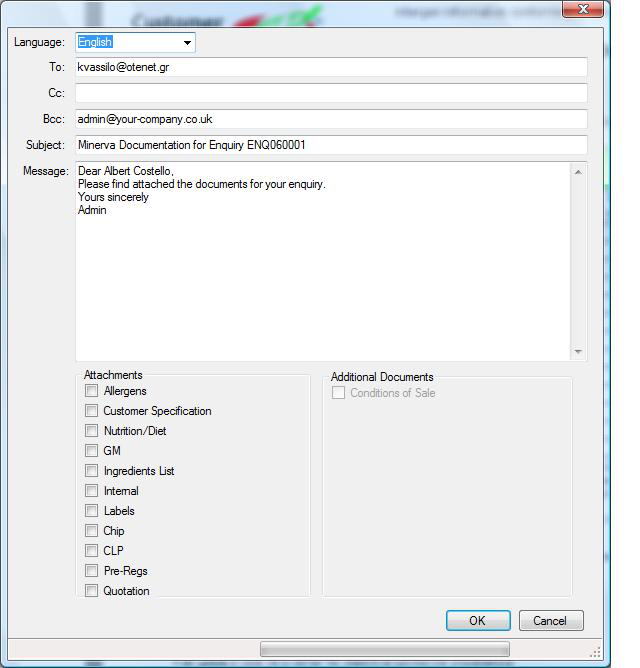 |
|
Select the reports to be printed and the language required with the printer to use, then click the button.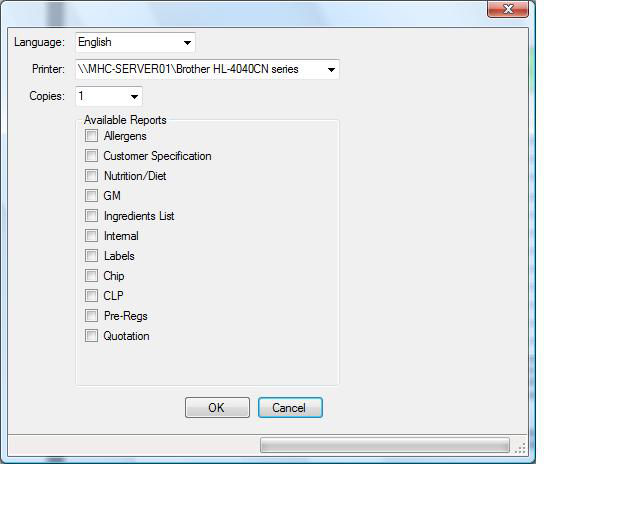 |
|
| When the formulation has been authorised you can use this button to return the formulation to the requirement tab. | |
| When the formulation has been authorised you can use this button to return the formulation to the formulation tab. | |
| This will return you to the quotation tab and un-authorise the formulation. | |
| This will set the status of the formulation as complete, but will not move the formulation to the Archive list. | |